Which One of the Following Orbitals Can Hold Two Electrons?
Pauli Exclusion Principle
- Page ID
- 660
The Pauli Exclusion Principle states that, in an atom or molecule, no ii electrons tin have the same four electronic quantum numbers. Equally an orbital tin can contain a maximum of only two electrons, the two electrons must have opposing spins. This means if i electron is assigned as a spin upwardly (+1/two) electron, the other electron must be spin-down (-ane/2) electron.
Electrons in the same orbital have the same starting time three quantum numbers, east.thousand., \(north=i\), \(l=0\), \(m_l=0\) for the 1south subshell. Only 2 electrons can have these numbers, so that their spin moments must be either \(m_s = -one/2\) or \(m_s = +1/ii\). If the anesouth orbital contains only one electron, we have 1 \(m_s\) value and the electron configuration is written as anes 1 (corresponding to hydrogen). If information technology is fully occupied, nosotros have two \(m_s\) values, and the electron configuration is ones 2 (corresponding to helium). Visually these two cases can be represented as
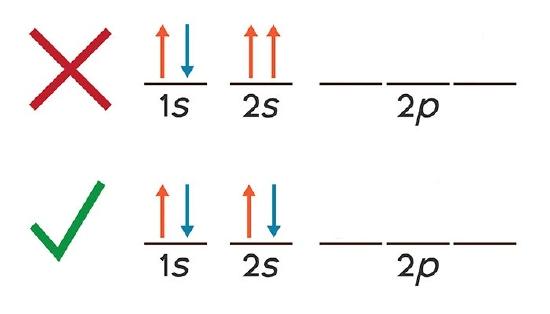
Equally y'all can see, the ones and 2s subshells for glucinium atoms can hold merely two electrons and when filled, the electrons must have opposite spins. Otherwise they will have the aforementioned four breakthrough numbers, in violation of the Pauli Exclusion Principle.
Contributors and Attributions
- Sarah Faizi (University of California Davis)
-
Dr. Craig Fisher (Nihon Fine Ceramics Eye)
Source: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_%28Physical_and_Theoretical_Chemistry%29/Electronic_Structure_of_Atoms_and_Molecules/Electronic_Configurations/Pauli_Exclusion_Principle
Posting Komentar untuk "Which One of the Following Orbitals Can Hold Two Electrons?"